Know about Emulsions
An emulsion is a fine mixture of two naturally immiscible liquids, most commonly water and oil. Emulsions are made possible by a special type of chemical that is called a surfactant or emulsifier, that is friendly to both liquids and which allows these two to coexist.
Emulsions are important in our lives, from protecting our lungs to making our skin feel great, to even harvesting pure oil from crude oil so we can use it as fuel. Emulsions allow for a variety of liquids, which can be used in numerous ways that benefit humanity. Let’s dive in to learn more about how emulsions form and what they can do!
Deepen your knowledge...
Chemistry of Emulsions
Emulsions are a specific type of colloid, or a mixture where microscopic particles are dispersed — without being dissolved — throughout another substance. An emulsion is a specific type of colloid because all the components of an emulsion are liquids.
In creating lotion emulsions, we are combining oil-based liquids and water-based liquids such that the end product appears as a uniform substance. This typically requires a rigorous mechanical force to shear and disperse one liquid into the other. Another example of this type of mixture lies in the kitchen when making a vinaigrette. To effectively combine water-based vinegar with oil, we might use a whisk or blender. In the case of lotions, we can employ a tool such as a stick blender.
Anyone who has combined oil and water knows that, regardless of how much you mix and shake, the vinaigrette will quickly separate into the oil phase (top) and water phase (bottom). This is because vinegar and oil are chemically incompatible. This is true for the water and oil components that we are working to combine in a lotion. Let’s explore why this is the case.
Emulsifiers and Surfactants
Stabilizing emulsions with surfactants
Let’s go back to making a vinaigrette. We might shake vigorously to mix oil and water together, but for the reasons mentioned above, the mixture will quickly separate into two distinct phases.
However, there are many contexts that require the stabilization of oil and water mixtures. How can we lower the energy barrier to prevent separation? The answer lies in the addition of emulsifying agents, also known as surfactants.
Surfactants are molecules or molecular compounds that lower the surface tension at an interface (interfacial tension). In the case of water-oil interface, adding a surfactant will lower the energy barrier for breaking hydrogen bonds among water molecules, and allow more freedom for water molecules to interact with other types of molecules in a given system. Additionally, surfactants have the ability to form micelles, which are a type of molecular “cage” that forms around solute molecules that are normally immiscible in a particular solvent (ie nonpolar molecules can be protected inside a micelle when immersed in a polar solvent).
From a chemical perspective, surfactants solve the incompatibility between water
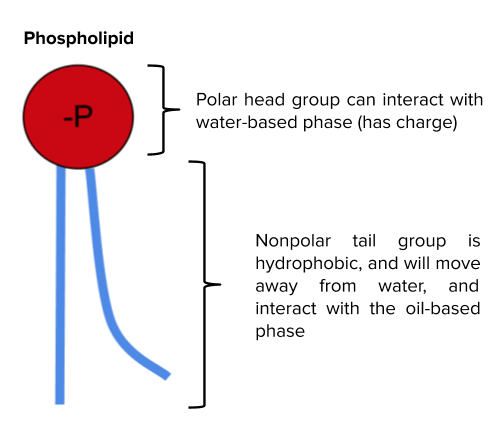 and oil because they contain both polar and nonpolar properties, serving as a bridge between polar/aqueous and nonpolar/organic substances. A common surfactant is a phospholipid, such as lecithin, which contains a polar headgroup that interacts with water, and a nonpolar tail that interacts with oils. In an emulsion, a phospholipid would surround the dispersed droplets, creating a micelle, forming a barrier between the water and oil.
and oil because they contain both polar and nonpolar properties, serving as a bridge between polar/aqueous and nonpolar/organic substances. A common surfactant is a phospholipid, such as lecithin, which contains a polar headgroup that interacts with water, and a nonpolar tail that interacts with oils. In an emulsion, a phospholipid would surround the dispersed droplets, creating a micelle, forming a barrier between the water and oil.
This presence of a phospholipid stabilizes the emulsion, preventing the oil and water components from separating. As described above, phospholipid molecules can help form a “molecular cage” to allow for oil phases to be separate from water phases, as shown in the schematic below.
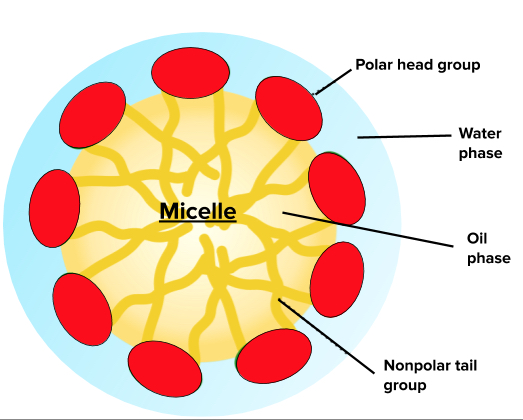
Characteristics of an Emulsion
As mentioned above, an emulsion is a type of colloidal mixture where normally immiscible liquids are combined in a way that maintains their unique chemical identities. In general, there are two parts of an emulsion:
Continuous Phase: the liquid portion of an emulsion in which another liquid is dispersed
Dispersed Phase: the liquid portion that forms tiny droplets that are evenly suspended throughout the continuous phase










